Osteoarthritis is a Systemic Disease: How High Blood Sugar and Adipokines Fuel Joint Breakdown
For decades, doctors dismissed osteoarthritis as inevitable wear and tear. But groundbreaking 2024-2025 research from Nature Reviews Rheumatology proves this “joint disease” actually starts in your bloodstream.
You’ve been told to lose weight and accept the pain. But no one explained why your friend with the same weight has perfect joints. Or why your hand arthritis gets worse after meals. The truth is more complex—and more actionable—than “just aging.”
Here’s what you’ll learn. How high blood sugar damages cartilage even without diabetes. The inflammatory molecules from fat tissue that attack your joints. Why osteoarthritis is now called a systemic metabolic disease.
Evidence-based treatments that target root causes. And the latest research showing real hope—including the STEP-9 trial that changed everything.
The Paradigm Shift: Why Osteoarthritis Is Now Called a Systemic Disease
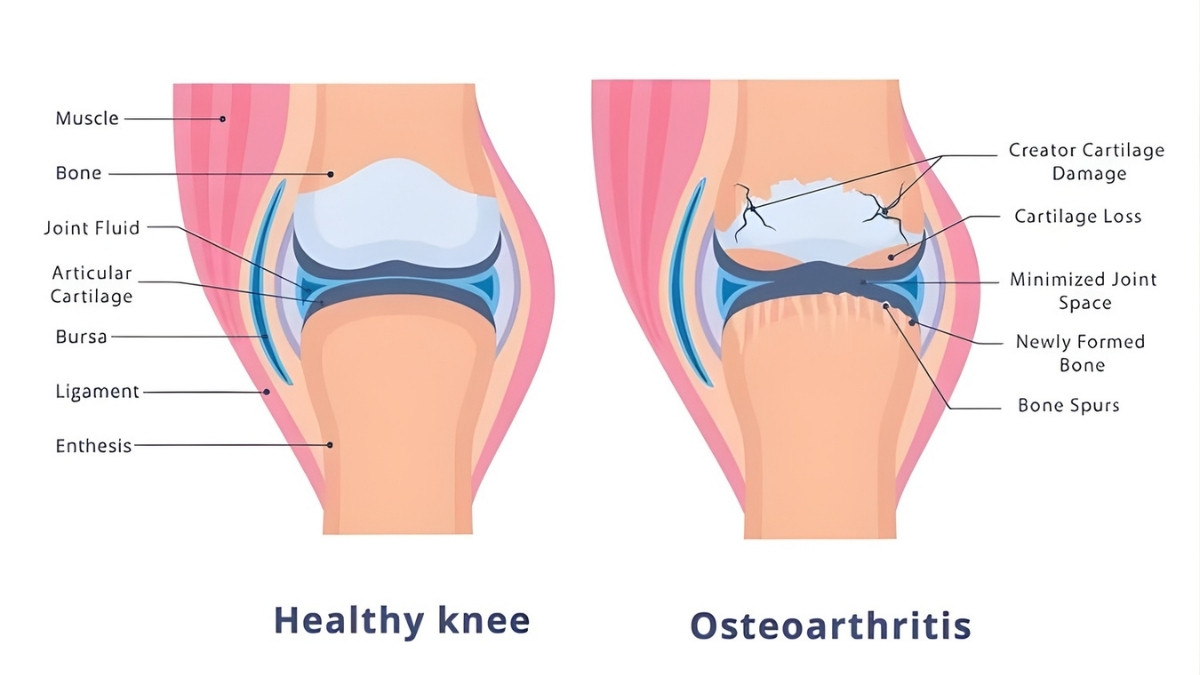
Doctors used to think osteoarthritis came from joints wearing out. Like tires on a car. But December 2025 research in Nature Reviews Rheumatology proved them wrong.
Here’s the evidence that changed everything. Obese people get hand and wrist arthritis. These joints don’t bear weight. If OA was just mechanical wear, this wouldn’t happen. But it does. This proves metabolic factors matter more than we thought.
The numbers tell the story. 52% of people with type 2 diabetes develop OA. Only 27% without diabetes get it. That’s not a coincidence. Right now, 595 million people globally have OA. By 2050, that number hits 1 billion. Your risk goes up 35% for every 5-point increase in BMI.
A systemic disease affects your whole body, not just one spot. Osteoarthritis is now officially in this category. Your bloodstream carries inflammatory signals to all your joints. This is why understanding OA as a systemic disease matters. It opens up new treatment options you’ve never heard about.
How High Blood Sugar Destroys Cartilage: The AGE-RAGE Connection
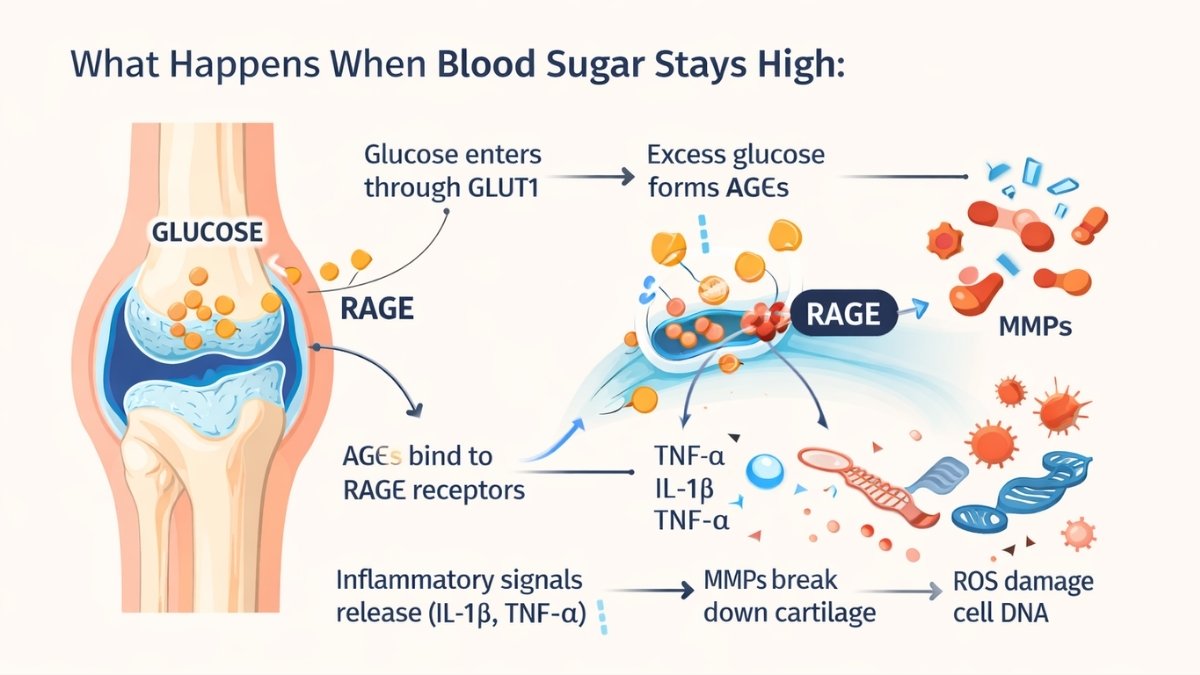
Your cartilage cells have glucose transporters called GLUT1. They let sugar in for energy. Normal amounts work fine. But when blood sugar stays high, these transporters become weapons.
Here’s what happens. Excess glucose enters your cartilage cells. It sticks to proteins permanently. These sticky proteins are called Advanced Glycation End Products—AGEs for short. Think of rust forming on metal. AGEs do the same thing to your cartilage. They make it stiff and brittle.
AGEs bind to receptors called RAGE. This triggers an inflammatory explosion. Your cells release TNF-α and IL-1β. These signals tell your body to break down cartilage. Enzymes called MMPs (matrix metalloproteinases) start destroying the cushioning in your joints. At the same time, Reactive Oxygen Species damage your cell DNA and proteins.
The research from Experimental & Molecular Medicine (June 2022) showed something scary. OA cartilage cells can’t turn down their glucose transporters. Even in high-sugar environments. This means constant damage. Diabetes patients have 20% higher rates of joint replacement surgery. Fasting glucose levels directly match knee damage on MRI scans.

What Happens When Blood Sugar Stays High:
- Glucose enters through GLUT1
- Excess glucose forms AGEs
- AGEs bind to RAGE receptors
- Inflammatory signals release (IL-1β, TNF-α)
- MMPs break down cartilage
- ROS damage cell DNA
Adipokines: The Inflammatory Molecules Destroying Your Joints
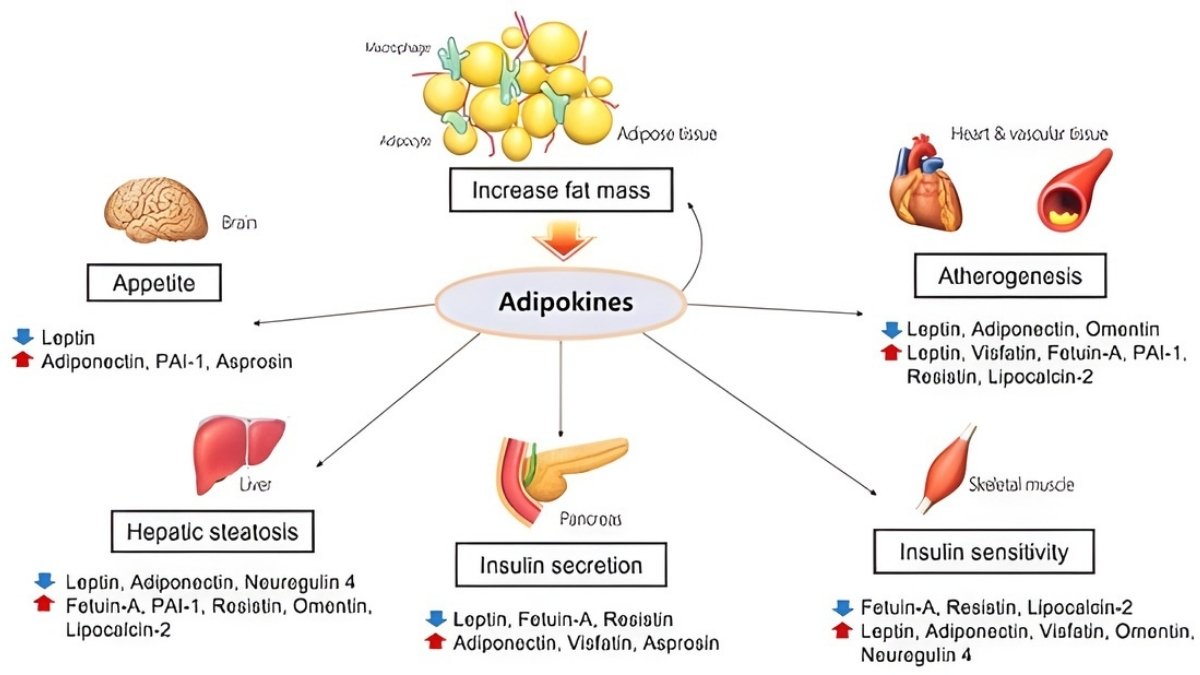
Your fat tissue isn’t just storage. It’s an active organ pumping out hormones called adipokines. These molecules travel through your blood to your joints. And they cause serious damage.
The main players are leptin, adiponectin, resistin, and visfatin. Studies show adipokine levels are “distinctly higher” in OA patients compared to healthy people. These molecules predict cartilage loss on MRI scans.
Higher leptin levels mean higher chances of needing knee replacement surgery. Synovial fluid—the liquid in your joints—shows adipokine levels that directly match pain severity.
Leptin: The Pain Amplifier
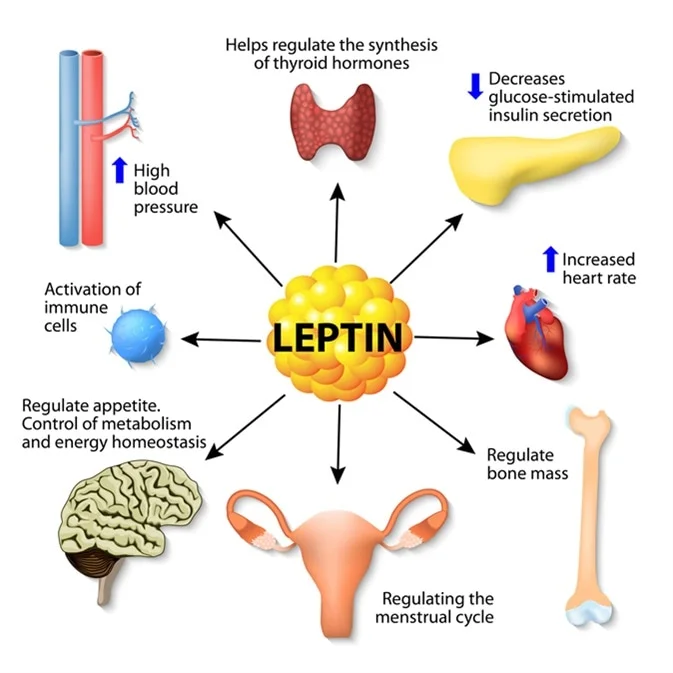
Leptin is a 16-kDa protein your fat cells make. More body fat means more leptin. This molecule stimulates IL-6, IL-8, and TNF-α in your joint tissues. These are inflammatory signals that cause pain and swelling.
Leptin hits cartilage cells directly. It triggers pathways that break down the cushioning matrix. Women typically have higher leptin levels than men. This partly explains why women get OA more often. The more you weigh, the more leptin you make. And the more your joints suffer.
Adiponectin: The Paradox Molecule
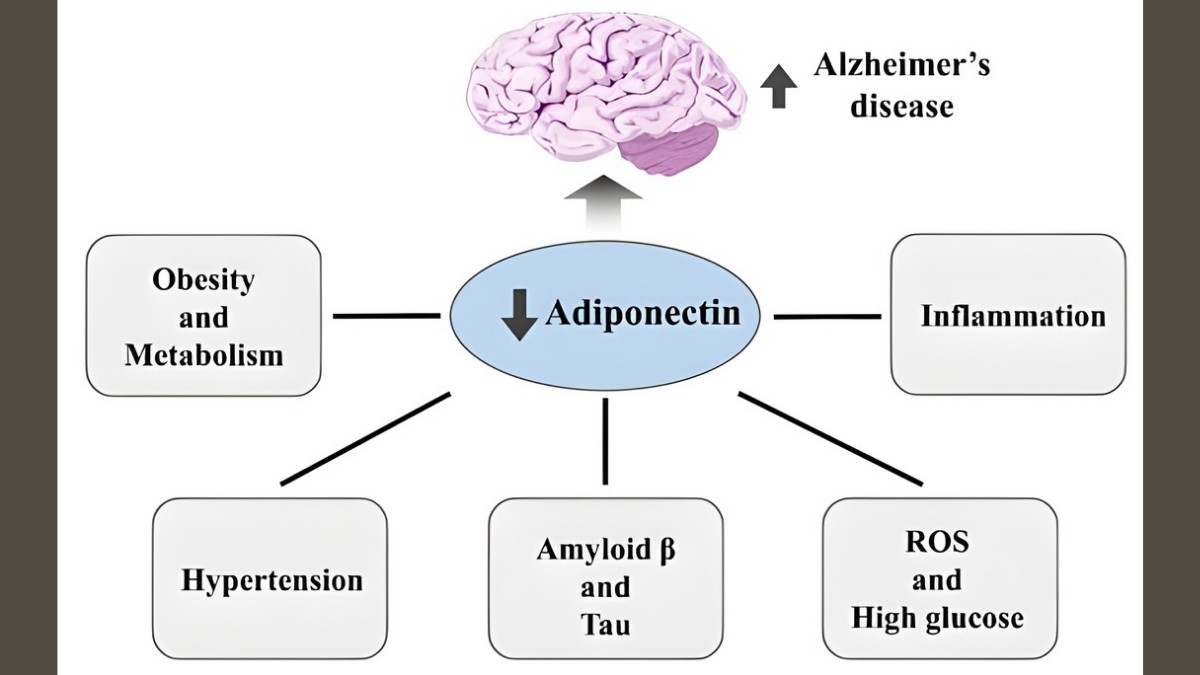
Adiponectin seems helpful at first. At low levels, it reduces inflammation. But in OA, something changes. The joint environment turns adiponectin from friend to enemy.
In arthritic joints, adiponectin increases MMP-1 and MMP-3 production. These enzymes chew up cartilage. Research shows adiponectin correlates with aggrecan degradation—that’s the main cushioning protein in your joints breaking apart. Higher adiponectin doesn’t protect you. It makes things worse.
The Cumulative Effect
Multiple adipokines work together. They create both local joint inflammation and whole-body inflammation. The infrapatellar fat pad—a fat deposit in your knee—pumps adipokines directly into the joint space.
This explains why both weight-bearing joints (knees, hips) and non-weight-bearing joints (hands, wrists) get damaged. It’s not about pressure. It’s about metabolic signals traveling through your bloodstream. Your hand arthritis and knee pain share the same root cause.
The Vicious Cycle: How Metabolic Syndrome Accelerates OA Progression
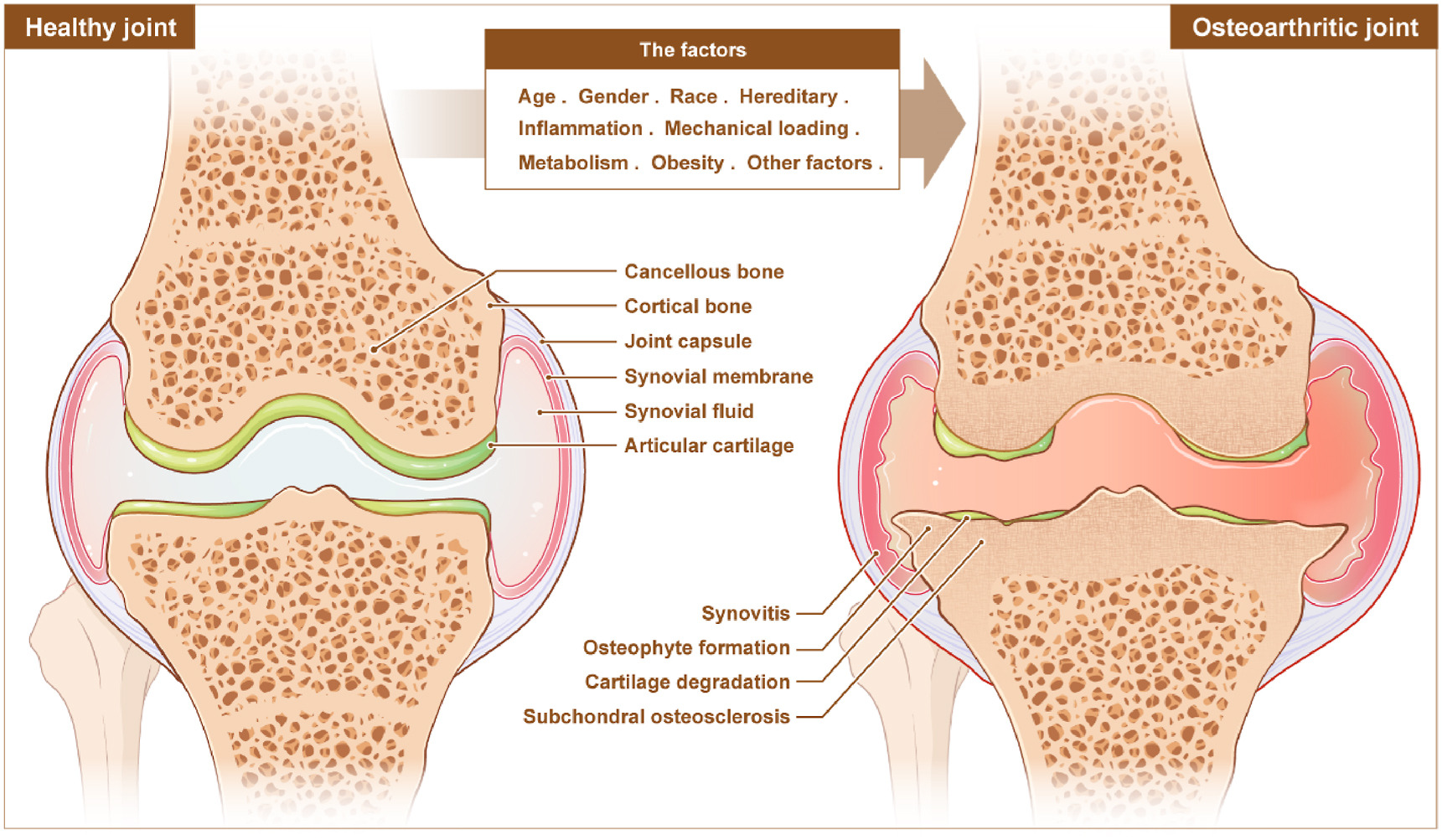
Metabolic syndrome includes obesity, high blood sugar, bad cholesterol, and high blood pressure. Each component damages your joints. Together, they create a cycle that feeds itself.
Insulin resistance doesn’t just affect diabetes. It affects your joints too. Type 2 diabetes causes damage through oxidative stress and chronic inflammation.
Studies on diabetic mice showed increased cartilage damage and synovial inflammation after just 10 weeks. Human patients with diabetes report worse pain on WOMAC scores—a standard arthritis pain measurement.
Here’s the cruel cycle. Joint damage makes you less active. Less activity worsens insulin resistance. Worse insulin resistance creates more inflammation. More inflammation damages joints further. This bidirectional relationship makes treatment harder.
Why Your Hand Arthritis Proves It’s Not Just Weight:
Hand osteoarthritis in obese people proves mechanical loading isn’t the whole story. Your hands don’t carry your body weight. But metabolic factors in your bloodstream reach ALL joints. This is why controlling your metabolism matters more than you think.
Evidence-Based Interventions: Targeting Metabolic Osteoarthritis in 2025
October 2024 changed everything. The New England Journal of Medicine published the STEP-9 trial results. This study proved we can actually treat the root cause of OA.
The STEP-9 Breakthrough: Semaglutide for Knee OA
407 people with obesity (BMI over 30) and moderate knee OA joined this 68-week study. Half got semaglutide 2.4 mg weekly injections. Half got placebo. The results shocked researchers.
Semaglutide group lost 13.7% of their body weight. Placebo group lost only 3.2%. But weight loss wasn’t the big news. Pain scores dropped 41.7 points on the WOMAC scale for semaglutide. Only 27.5 points for placebo. That’s a 14-point difference beyond weight loss alone.
Physical function improved 12.0 points versus 6.5 points. The Shanghai OA Cohort backed this up. GLP-1 users lost 7.29 kg. Only 1.7% needed surgery versus 5.9% in the control group. Cartilage loss slowed by 0.02 mm (p=0.004). This means semaglutide might have direct anti-inflammatory effects on joints—not just weight loss benefits.
Dietary Strategies That Work

Food choices directly affect your joints. A 2025 meta-analysis showed dietary interventions reduce pain with an effect size of -0.67. That’s clinically meaningful.
Low-glycemic diets reduce AGE formation. Choose steel-cut oats over instant. Pick sweet potatoes over white bread. Eat beans and lentils. These foods keep blood sugar stable.
Anti-inflammatory foods help too. Fatty fish like salmon provide omega-3s. Leafy greens, berries, and colorful vegetables fight inflammation. Mediterranean diet patterns show promise in recent studies.
Foods to avoid: refined carbohydrates, saturated fats, and added sugars. People eating the most added sugar have 40% higher OA rates. Skip the soda. Ditch the candy. Your joints will thank you.
Blood Sugar Control Beyond Medication

HbA1c measures your 3-month average blood sugar. Lower is better for joints. Continuous glucose monitors help you see patterns. You’ll spot foods that spike your sugar.
Exercise works double duty. It controls glucose AND maintains joints. Resistance training preserves lean muscle mass. This improves your metabolic health. Walk, swim, lift weights—whatever you can do consistently.
Metformin’s Emerging Role
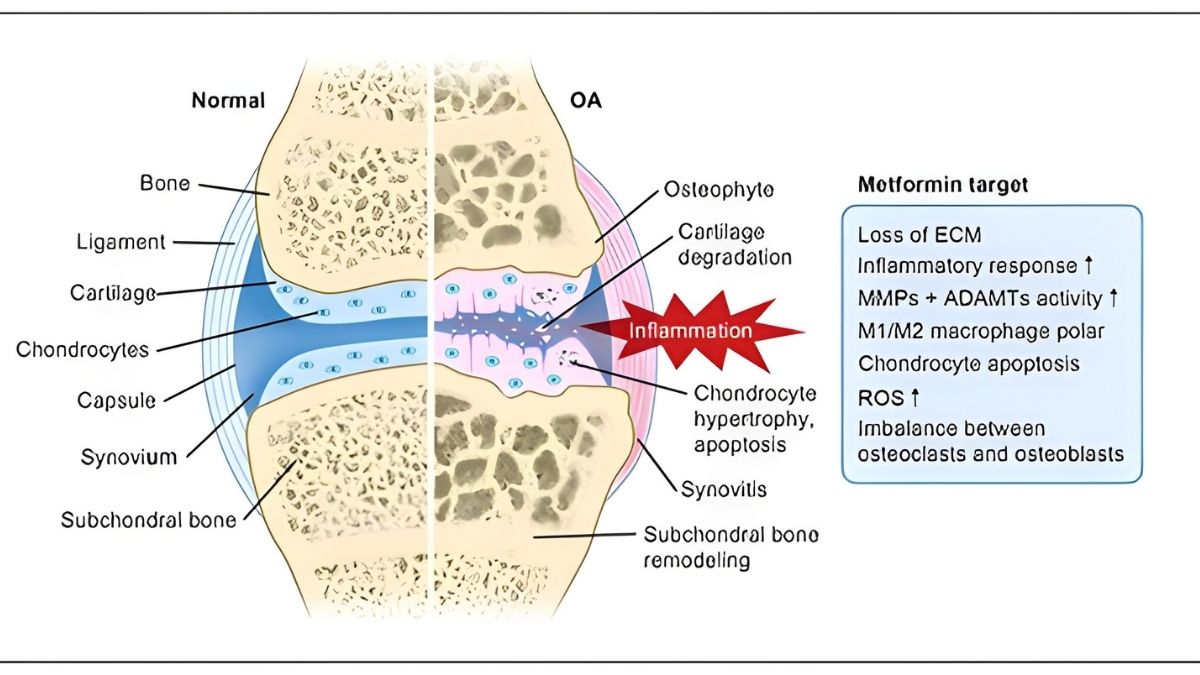
Metformin is an old diabetes drug showing new promise for OA. A network meta-analysis ranked it highest for pain relief among medications studied. It activates AMPK pathways and reduces inflammation.
The safety profile looks good in OA patients with or without diabetes. Research is ongoing. Talk to your doctor about whether metformin makes sense for you.
Your 4-Week Metabolic OA Action Plan:
- Week 1: Monitor blood sugar, cut added sugars
- Week 2: Add fatty fish and leafy greens
- Week 3: Switch to low-glycemic eating
- Week 4: Discuss GLP-1 or metformin with doctor
Prevention Strategies: Protecting Your Joints Before Metabolic Damage Starts
The best time to act is before damage begins. Prediabetes is your critical window. This is when fasting glucose sits between 100-125 mg/dL. You’re not diabetic yet. But your joints are already at risk.
Body composition matters more than weight alone. High fat mass plus low lean mass emerged as the critical factor in 2025 research. Two people can weigh the same. The one with more muscle and less fat has healthier joints.
Every 10% weight reduction provides significant symptom relief. But you need to lose the right kind of weight—fat, not muscle. Physical activity reduces insulin resistance AND supports joint health. Aim for 150 minutes of moderate activity weekly.
Early screening catches problems before they’re visible on X-rays. Check your fasting glucose. Measure inflammatory markers like hs-CRP. Get a lipid panel. These simple blood tests reveal your risk level.
Risk Assessment: Are You at Risk for Metabolic OA?
- □ BMI over 30
- □ Prediabetes or diabetes diagnosis
- □ Fasting glucose over 100 mg/dL
- □ Metabolic syndrome diagnosis
- □ Family history of early OA
- □ Hand or wrist pain
- □ Post-meal blood sugar spikes
If you checked 3 or more boxes, you need aggressive metabolic intervention now. Don’t wait for severe pain.
Conclusion:
Osteoarthritis isn’t inevitable aging. It’s a systemic metabolic disease driven by high blood sugar and inflammatory adipokines. The STEP-9 trial proved we can target root causes—semaglutide showed 41.7-point pain improvements.
Check your fasting glucose. Assess your metabolic health. Discuss interventions with your doctor. Understanding osteoarthritis as a systemic disease transforms it from inevitable decline to a manageable condition. The choice to act is yours.